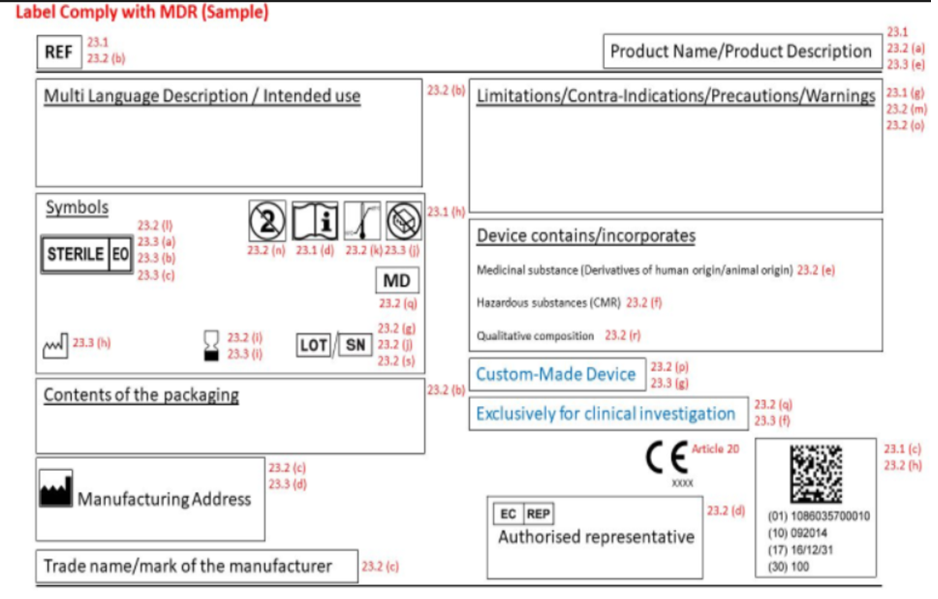
Medical Device Labelling
Medical Device Labelling is the means to communicate information related to the safety and the performance of the device to the users which includes the proper identification of the device or group of devices too. It helps the end user to use the device as intended by the manufacturer.
Labelling includes
- User manual
- Service Manual
- Package inserts
- Primary package label
- Shipping Label
- Sales Literature
- Website pages
- Marketing advertisements
- Instruction for Use etc